The gene transfer therapy process begins when a working (or functional) gene is created in the laboratory. The working gene is developed to contain the instructions for making a needed protein.
Scientists design working genes to meet specific needs. For example, in patients with hemophilia A, the F8 gene is needed to code for the factor VIII protein, which is essential for clotting, and in hemophilia B, an F9 gene is needed to code for the factor IX protein.

The working gene now has to be delivered into the body. It needs to be protected and directed on where to go. To do so, a therapeutic vector is used. This therapeutic vector is created by modifying a naturally occurring virus; the shell of the virus is created without the viral DNA and the working gene is put inside the empty shell. No longer a virus, the therapeutic vector is designed to deliver the working gene to the cells in the body where it is needed.

The working gene DNA is protected and transported into the body via a therapeutic vector.
Like a working gene, a therapeutic vector is created in the laboratory. Trillions of vectors are created by scientists who build the empty outer shell of the virus. Because the body has a natural defense against external proteins, the shell is needed to protect the working gene and serves as a transport vehicle to guide it to the proper cells within the body. The viral shell is sometimes called a neutralized virus because viral DNA is absent.
As part of gene therapy research, a healthcare provider must determine whether a patient is eligible. Factors such as age, gender, and organ health may be considered.
Additional eligibility criteria
Therapeutic vectors being used in research are commonly made from adeno-associated viruses (AAVs). These viruses are not known to make people sick. They are found naturally around the world, so some people will have already developed immunity to them via exposure at some point in the past. Having pre-existing immunity to the AAV used in gene therapy could reduce or eliminate its effectiveness. Because of this, candidates may have to be screened with a blood test to ensure that they do not have immunity.

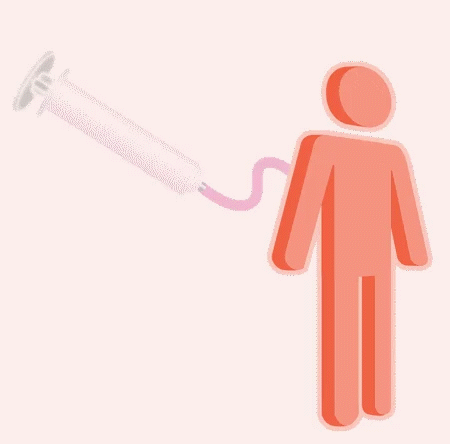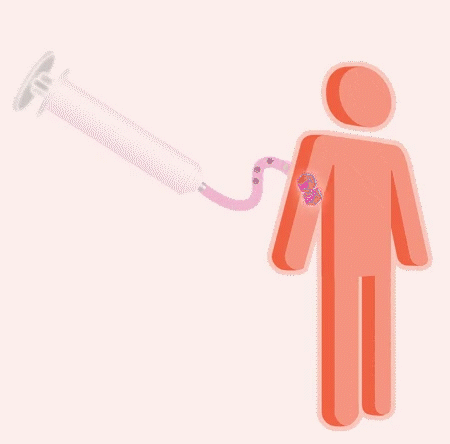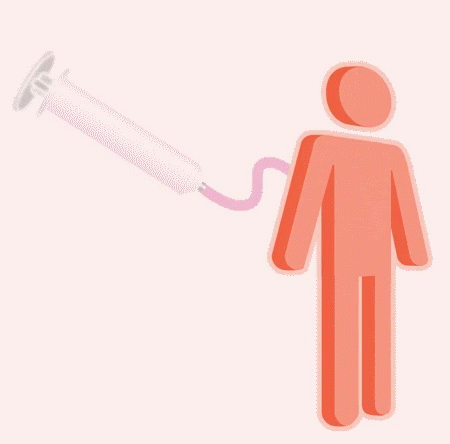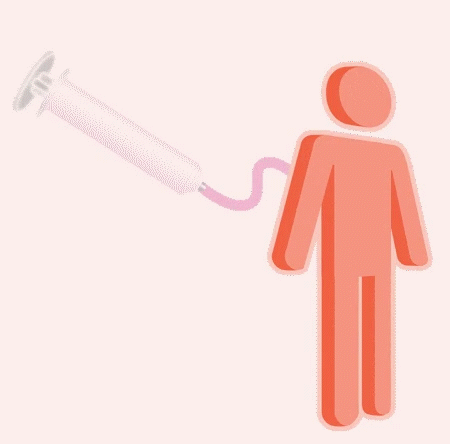
Once the patient is determined to be eligible, the gene therapy is ready for administration to evaluate its safety and impact.
A single, one-time infusion in an appropriate clinical setting delivers large numbers of therapeutic vectors into the body. The therapeutic vector is designed to both protect and guide the working gene toward preferred cells where it can be used to make the needed protein. Research is ongoing to determine the possible impact of the therapeutic vector delivering the working gene to other cells in the body.
Once in the body, the new gene is designed to do the work of the gene that is missing or isn’t functioning properly. The goal is to provide instructions for the body to make the protein it needs on its own, and ongoing research is evaluating the risks and impact of introducing the new gene.
Does gene therapy replace the mutated or non-working gene?
Because the new, working gene is not intended to become part of your DNA, the original missing or mutated gene is left unchanged. Gene transfer therapy is not designed to replace or edit the existing gene, which means that the mutated gene could still be passed to future generations.
The specific vectors used in gene therapy are chosen because of their ability to target appropriate cells within the body. In gene transfer therapy for hemophilia A and B, vectors that target liver cells are being investigated because these cells can make the proteins required for blood to clot.
It’s an exciting time in science and things are changing every day. Sign up and we’ll be sure to keep the conversation going.
Regular monitoring after gene therapy is important because it allows researchers to understand any risks and what impact the gene transfer is having. Patients in clinical trials meet with their care teams for blood tests and to discuss their medication regimen and lifestyle to collect data as part of the study.
As with all medications, response to gene therapy may vary. How long gene therapy might keep working is being evaluated in ongoing clinical trials with researchers aiming to create a long-lasting therapy.
Many gene therapies for hemophilia are being studied in people to determine if they are tolerable and effective. This educational website will not focus on any commercially available products or specific gene therapies being researched for hemophilia A or B.
Sign up for information on upcoming events, new resources, and the latest in gene therapy research.